
Expanding pharmaceutical products into the United States is a high-value opportunity, but it comes with one of the world’s most stringent regulatory frameworks. The U.S. Food and Drug Administration (FDA) enforces strict compliance across drug safety, efficacy, labeling, and manufacturing quality. For Indian and global pharmaceutical companies, working with a US FDA Drug Registration Consultant in India is the most reliable way to navigate this complex regulatory environment
Understanding US FDA Drug Registration
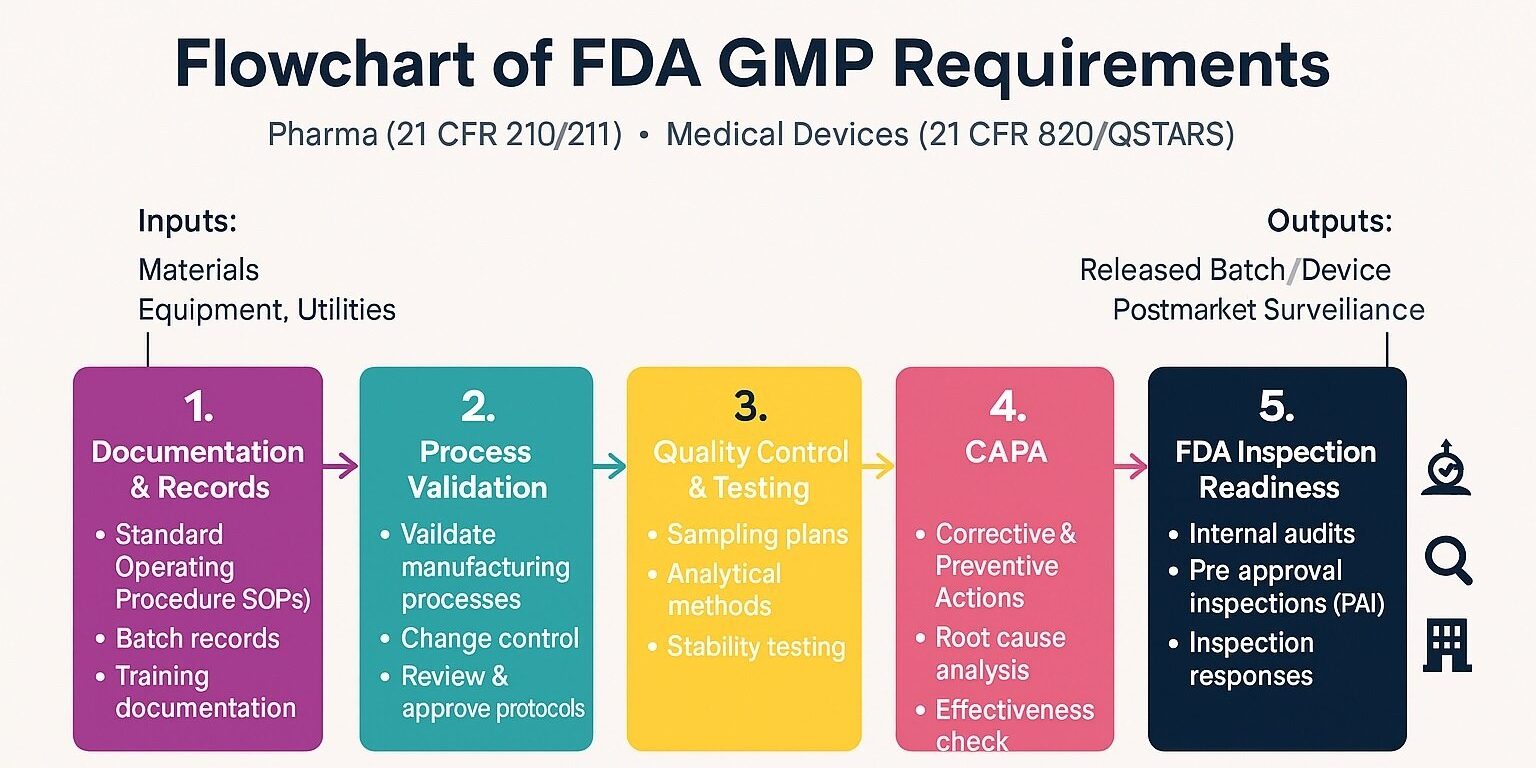
US FDA drug registration is the mandatory regulatory process that pharmaceutical companies must complete before manufacturing, exporting, or selling drug products in the United States. The FDA evaluates whether a drug meets defined standards of quality, safety, and effectiveness, and whether the manufacturing facility complies with current Good Manufacturing Practices (cGMP).
Registration is not a single step—it is a lifecycle obligation involving:
- Facility registration and renewal
- Drug product listing
- Regulatory submissions (NDA, ANDA, IND, OTC)
- FDA inspections and audits
- Post-approval compliance and reporting
A professional consultant ensures that these obligations are managed accurately and continuously.
Why Indian Pharmaceutical Companies Need US FDA Consultants
India is one of the largest suppliers of pharmaceutical products to the US market. However, FDA scrutiny of overseas manufacturers is extremely rigorous. Even minor documentation errors or compliance gaps can result in import alerts or warning letters.
A US FDA Drug Registration Consultant provides:
- Local regulatory coordination with US compliance expertise
- Accurate interpretation of FDA regulations
- Proactive inspection readiness
- Structured FDA communication and response handling
XPRO America, a US FDA Consultancy, bridges the regulatory gap between Indian manufacturers and US FDA expectations.
US FDA Drug Regulatory Pathways Explained
Selecting the correct regulatory pathway is fundamental to successful FDA registration. Each pathway has unique data, timeline, and cost implications.
NDA – New Drug Application
For novel drugs or new combinations requiring extensive clinical evidence.
ANDA – Abbreviated New Drug Application
For generic drugs demonstrating bioequivalence with an approved reference drug.
IND – Investigational New Drug
Required before initiating clinical trials in the United States.
OTC Drug Registration
For non-prescription drugs, either under OTC monographs or via NDA where applicable.
An experienced FDA consultant ensures the pathway selection is accurate, preventing costly re-submissions.
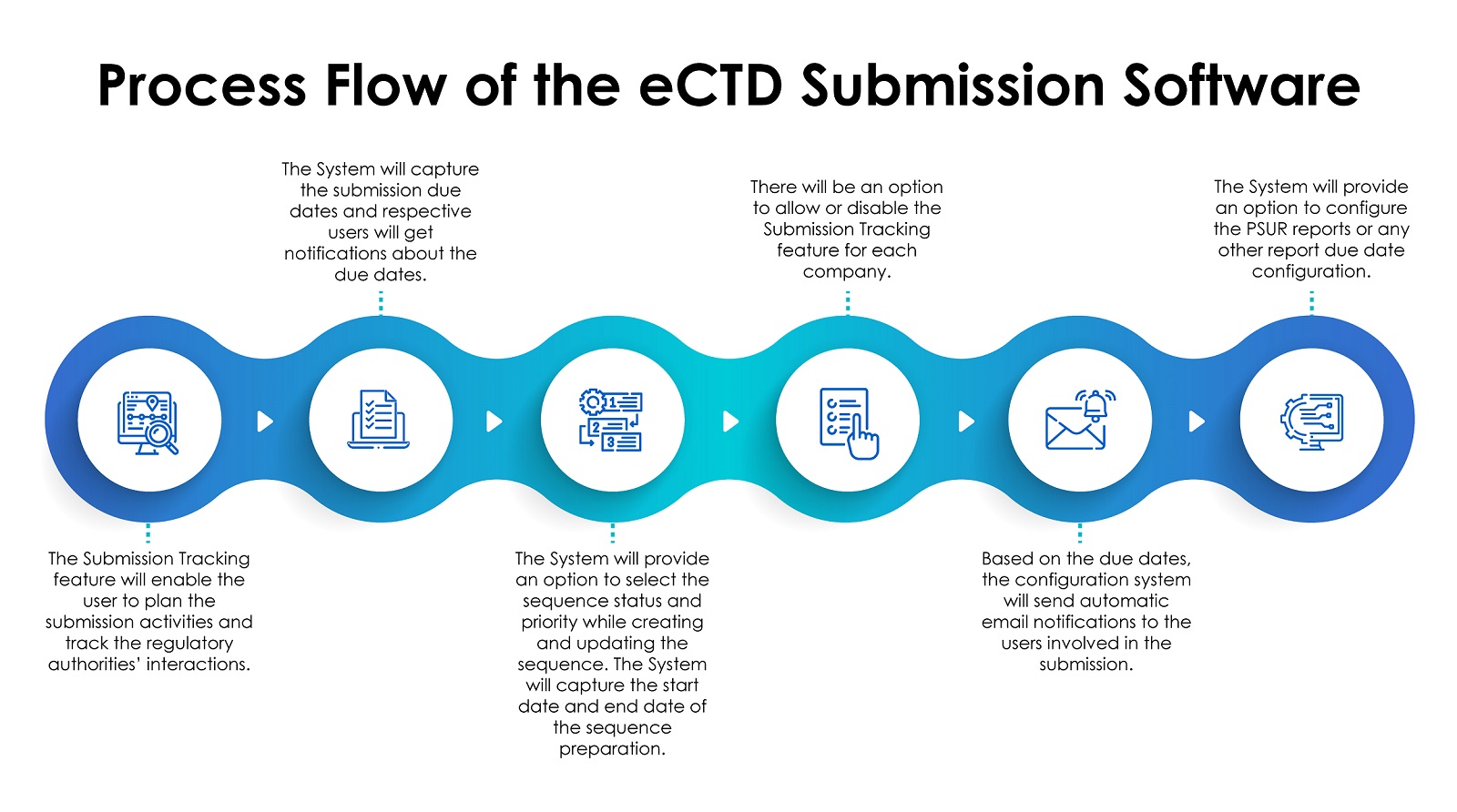
End-to-End US FDA Drug Registration Process

Step 1: Regulatory Assessment & Gap Analysis
Evaluation of product type, formulation, intended use, and existing compliance gaps.
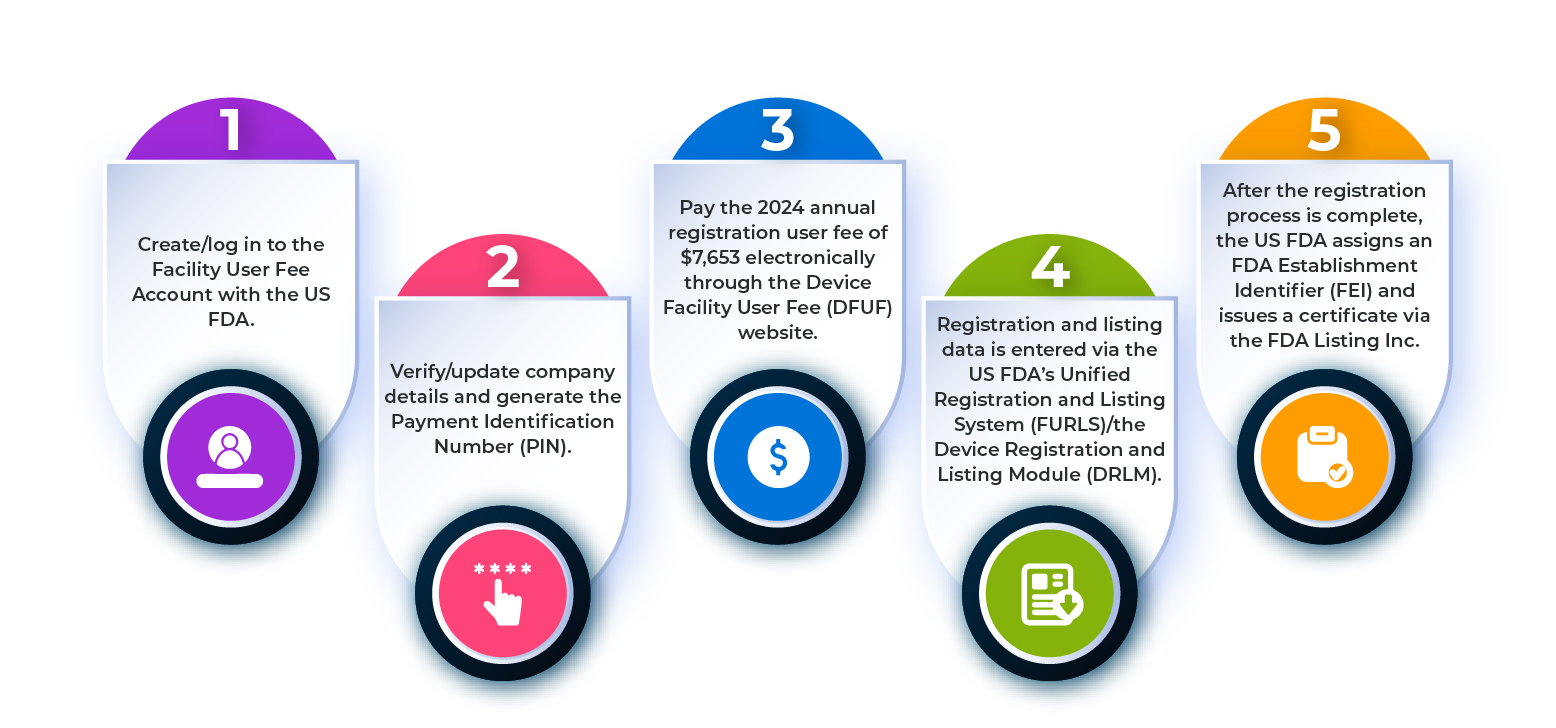
Step 2: FDA Establishment Registration
Mandatory registration of manufacturing, testing, packaging, and labeling facilities with annual renewals.
Step 3: Drug Product Listing
Each drug is listed with the FDA, linked to its registered facility and assigned appropriate identifiers.
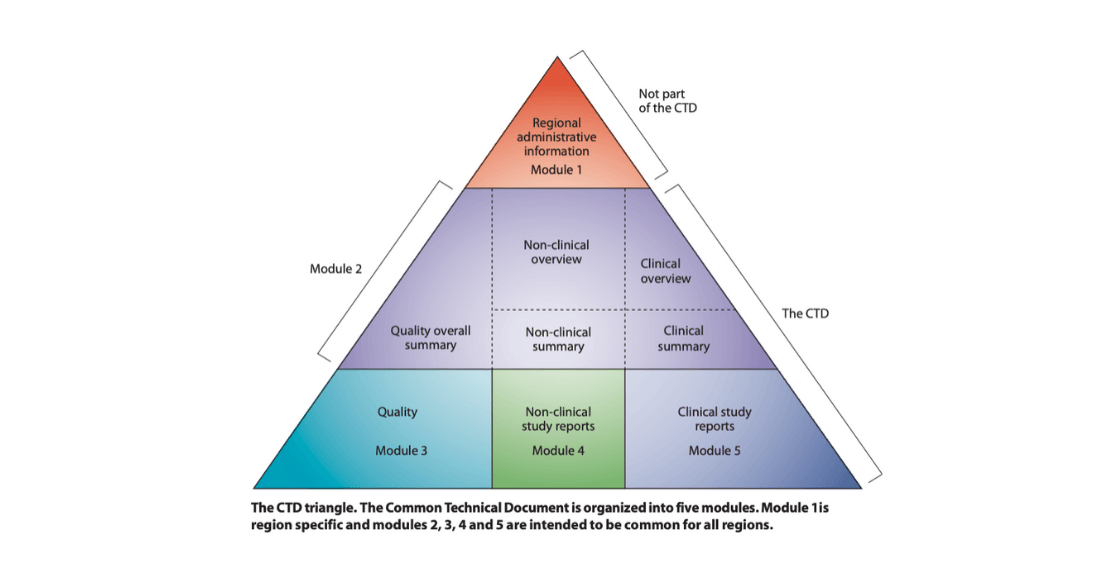
Step 4: Dossier Preparation (eCTD)
Complete technical documentation is prepared in FDA-mandated electronic Common Technical Document (eCTD) format, including CMC, stability, and clinical/bioequivalence data.
Step 5: FDA Submission & Review
Applications are reviewed by FDA scientists and reviewers, with formal queries issued if clarification is required.
Step 6: FDA Inspection & Audit Support
Facilities may be inspected for cGMP compliance. Consultant-led preparation significantly reduces inspection risks.
Step 7: Approval & Lifecycle Management
After approval, ongoing compliance includes adverse event reporting, labeling updates, and periodic FDA interactions.
XPRO America, a US FDA Consultancy, manages every stage of this process with precision.
Common Compliance Risks Without a Consultant
Pharmaceutical companies attempting FDA registration independently often face:
- Incorrect eCTD structure
- Inadequate CMC documentation
- Delayed or incorrect FDA responses
- Inspection observations (Form 483)
- Import alerts and product detention
A qualified consultant anticipates these risks and implements corrective strategies early.
XPRO America – US FDA Consultancy for Drug Registration
XPRO America is a dedicated US FDA Consultancy supporting Indian and international pharmaceutical companies seeking US FDA drug approval.
Core Drug Registration Services:
- FDA drug regulatory strategy development
- NDA, ANDA, IND, and OTC submissions
- FDA establishment registration and drug listing
- eCTD publishing and validation
- FDA query and deficiency letter response
- US Agent services for foreign manufacturers
- Post-approval regulatory compliance management
XPRO America’s regulatory experts work closely with client technical teams to ensure accuracy and audit-ready documentation.
Benefits of Working with a US FDA Consultant in India
Partnering with XPRO America offers:
- Reduced approval timelines
- Lower regulatory risk
- Improved inspection outcomes
- Clear regulatory accountability
- Long-term FDA compliance assurance
These benefits directly translate into faster US market access and sustained commercial success.
Who Should Use US FDA Drug Registration Services?
US FDA consultancy services are essential for:
- Indian pharmaceutical exporters
- Generic and branded drug manufacturers
- API manufacturers entering finished dosage markets
- OTC drug companies
- Contract manufacturers serving US clients
Regardless of size, FDA compliance is mandatory for US market participation.
Importance of Ongoing FDA Compliance
FDA approval is not the end of regulatory responsibility. Non-compliance after approval can result in:
- Warning letters
- Import alerts
- Product recalls
- Loss of US market access
A professional consultant ensures continued adherence to FDA regulations throughout the product lifecycle.
Conclusion
US FDA drug registration is a highly regulated, documentation-intensive, and inspection-driven process. For Indian pharmaceutical companies, working with a knowledgeable consultant is not optional—it is strategic.
XPRO America, a US FDA Consultancy, provides structured, compliant, and result-oriented FDA drug registration services that help pharmaceutical companies enter and sustain success in the US market.
📧 For professional US FDA drug registration support, contact:
support@xproamerica.com
Leave a Reply